Industry Talk - Interviews
Shaping a Smoke-Free Future: PMI Lebanon’ s Amr Taha Speaks Out
by Ghada Azzi
October 1, 2025
.jpg) Advertisement
AdvertisementArabAd sat down with Amr Taha, the newly appointed General Manager of Philip Morris Lebanon, to discuss PMI’s vision, the science behind IQOS, and how the company navigates regulation and responsibility in a shifting tobacco landscape.
Given the significant number of smokers in Lebanon, how will PMI be involved in reducing this number and positively impacting health?
Philip Morris International and government and health agencies worldwide share a common goal: reduce smoking rates. In recent decades, we have seen marked progress resulting from government-funded prevention and cessation programs.
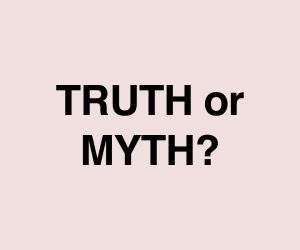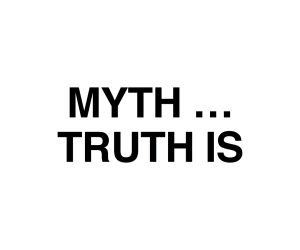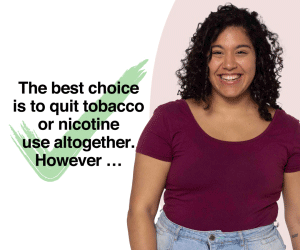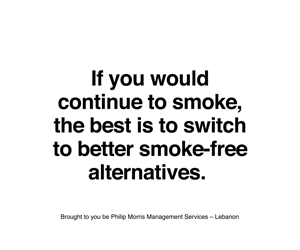
Nevertheless, around a billion people continue to smoke, and—given projected population growth—this number is unlikely to decline in the foreseeable future. We can move the needle and progress faster on reducing smoking and the associated harms by combining traditional tobacco control measures with support for the smoke-free alternatives now available.
By eliminating combustion in our heated tobacco products, we can reduce the exposure to harmful and potentially harmful substances by an average of 90-95% compared to traditional cigarettes. Although not entirely risk-free, this is still a better option for adults than continued smoking.
Our core strategy is to avail smoke-free alternatives to adult smokers who would otherwise continue to smoke.
Launching our heated tobacco products IQOS and BONDS involves ensuring availability and consumer awareness through clear information and expanded retail presence.
We also make our science available and transparent to encourage switching to these better alternatives for adult smokers.
On this occasion, I'd like to share that we constantly enhance and evolve our portfolio of smoke-free alternatives to support our vision, and that we have proudly brought to Lebanon IQOS Iluma i last month, which is the most advanced heat-not-burn technology we have developed yet.
ArabAd: PMI has emphasized the reduced-risk profile of IQOS. How does the company substantiate these claims?
Amr Taha: The breadth and depth of our IQOS studies are unprecedented in the to be cross-checked with industry.
We have conducted 18 nonclinical and 10 clinical studies, forming the bulk of a scientific evidence package submitted to regulators such as the U.S. FDA. We believe the totality of this evidence—clinical data, aerosol chemistry, and nonclinical results—demonstrates that switching completely to IQOS, while not risk-free, is a better choice for adults who would otherwise continue smoking.
AA: What measures are in place to ensure that the public accurately understands the health implications?
A.T: People who smoke deserve access to accurate, non-misleading information to make better decisions about their health. Science and evidence remain at the heart of our mission. That’s why, at PMI, we share our research with the scientific community, governments, and regulators to ensure transparency and accountability. We are also clear: these products are not risk-free but are a better alternative to cigarettes.
But to achieve a smoke-free future, all stakeholders—from governments to the scientific community—must recognize the role these products can play. Regulations should both discourage initiation and encourage quitting, while ensuring adult smokers have access to better alternatives.
“If you don’t
smoke, don’t start. If you smoke, quit. If you don’t quit, then switch to
better alternatives.”
AA: How does PMI balance business interests with public health considerations in regulatory discussions?
A.T: There is growing support for smoke-free alternatives. Public health advocates and regulators alike are beginning to embrace the public health potential of smoke-free alternatives. A growing number of public health institutions, experts, and governments support the role of potentially less harmful alternatives for adult smokers and public health.
There is growing support for smoke-free alternatives. Public health advocates and regulators alike are beginning to embrace the public health potential of smoke-free alternatives. A growing number of public health institutions, experts, and governments support the role of potentially less harmful alternatives for adult smokers and public health.
Among regulators that have taken initiatives to recognize the different nature of smoke-free products and set rules to provide smokers with access to and information about science-based less harmful alternatives, it is worth citing Bulgaria, E.U., Italy, Portugal, the U.S., and U.K. Others, like Greece, Norway, and New Zealand, have announced or are already taking steps in the same direction.
The U.S. FDA’s exposure modification order for IQOS confirms that IQOS is fundamentally different from cigarettes. It highlights the fact that all tobacco and nicotine-containing products are not the same, and that policy and regulation should reflect this.
AA: Given strict advertising regulations, how do you market IQOS responsibly while reaching adult smokers?
T: PMI is clear that if you don’t smoke, do not start. If you smoke quit. If you do not quit, then switch to better alternatives. People who have never used tobacco or nicotine products, especially minors, should not use any tobacco or nicotine products. Our smoke-free products are intended for only one audience: adults who smoke and who would otherwise continue to do so.
Our Marketing Code, which often goes further than local laws, serves as our bedrock for encouraging. adults who would otherwise continue smoking to switch to innovative products that do not burn tobacco, while working to guard against unintended use.
We believe that commercialization of these products should balance two essential needs: Adult smokers should receive accurate information about these better alternatives and their risks, and should be encouraged to switch if they would otherwise continue smoking. Nonsmokers and minors should be protected and should not use these products.
AA: There have been reports of PMI using social media influencers. How do you address concerns that such strategies might appeal to underage audiences?
A.T: PMI does not use social media influencers to promote its products. We refuse such partnerships and age-restrict our digital channels. We follow a strict internal marketing code, often stricter than local laws, to prevent youth appeal. Our communications are designed not to attract minors.
At PMI, our position is unequivocal: tobacco or nicotine products are not for individuals who have never used them—particularly minors.
Our smoke-free innovations are designed with a singular purpose: to offer adult smokers, who would otherwise continue smoking, a better alternative.
We design all our communications in a way that is not appealing to youth and implement strict measures to limit youth interest and access to our products. Our practices are governed by a set of internal rules and marketing standards, including our Good Conversion Practices, which are often more restrictive than local laws.
We also believe governments should monitor youth adoption and take swift corrective measures where necessary. Encouragingly, independent studies confirm that youth uptake of heated tobacco products, including IQOS, remains low.
Amr Taha’s message is clear: IQOS is no silver bullet, but it is a step toward harm reduction. Whether this resonates in Lebanon’s complex social and regulatory fabric is a question that only time—and consumers—will answer.
In the end, Philip Morris’s positioning balances on a delicate line: promoting innovation without seducing the wrong audience. For ArabAd, that’s where the story lies—not just in the science, but in the smoke and mirrors of perception, regulation, and culture.



.jpg)